Role of Dendritic Cells in Health and Disease
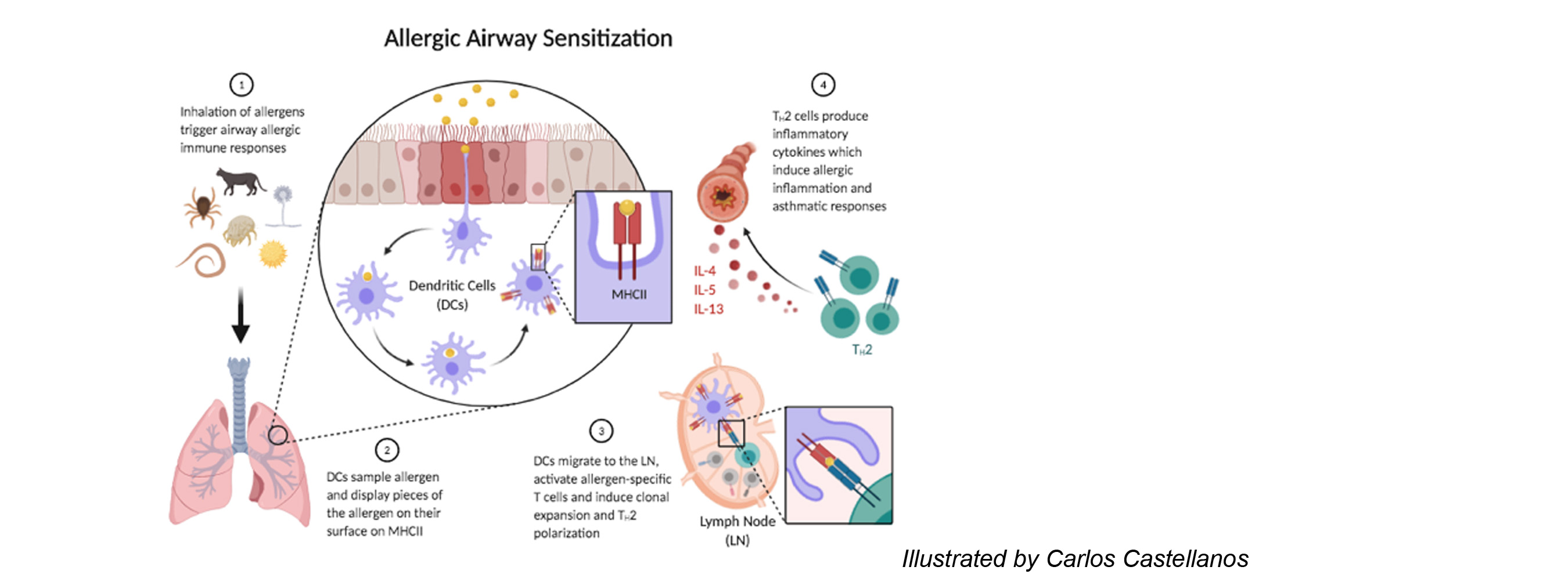
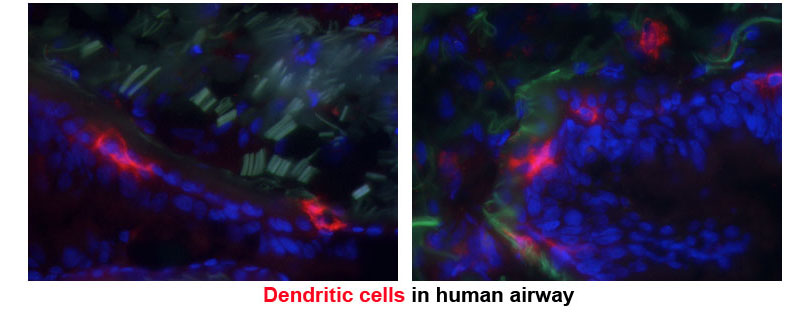
Dendritic cells (DCs) reside in peripheral tissues where they monitor tissue invasion by foreign antigens. Following encounter of antigens, DCs migrate to the draining lymph node and present antigens to naïve T cells inducing further differentiation. DCs that present antigens derived from virus or intracellular bacteria drive the differentiation of cytotoxic T lymphocytes or T helper type 1 (Th1) cells. These effector T cells produce soluble molecules that either directly kill the virus-infected host cells or assist macrophages and other innate immune cells to kill intracellular bacteria. DCs that present antigens derived from fungi or extracellular bacteria drive the differentiation of Th17 cells, which induce recruitment of neutrophils and macrophages to the site of infection and help these cells clear the microbes. DCs that present antigens derived from parasitic worms drive the differentiation of Th2 cells, which induce recruitment of eosinophils and other granulocytes and induce expulsion of the worms. Under certain conditions, DCs harm the host by driving unnecessary or aberrant T cell immunity to innocuous antigens. DCs that encounter allergens carry them to the lymph node, present them to allergen-specific T cells, and drive differentiation of pathologic Th2 cells. The cytokines produced by these Th2 cells bring eosinophils to the site of allergen exposure, which mediates inflammatory rash causing dermatitis and bronchial constriction triggering asthma. DCs can also present self-antigens to T cells and drive differentiation of pathologic Th1 or Th17 cells. These T cells produce cytokines that mediates tissue inflammation and destruction and significantly contributes to the development of autoimmune diseases.
Role of Membrane Trafficking in Dendritic Cell Function
Membrane proteins and their trafficking play an instrumental role in DC function of sensing and presenting antigens and driving T cell immunity. Many of microbial sensors are localized in the plasma membranes or endosomes and transmit intracellular signal to DCs upon binding of microbes. The antigen presenting molecules MHCI and MHCII load antigens derived from extracellular and intracellular antigens in endoplasmic reticulum and lysosome respectively, and traffic to the plasma membrane for presentation to T cells. The chemokine receptor CCR7 is constitutively internalized in resting DCs but recycled back to the plasma membrane upon binding by CCL19 and transmits intracellular signal that mediates DC migration to lymph nodes.
Our laboratory is interested in discovering novel membrane trafficking pathways in DCs, identifying the molecular mechanisms governing the trafficking, and exploiting these mechanisms to develop therapeutics for treatment of immune stimulatory diseases. Past research included trafficking of FceRI, MHCII, and CD86. FceRI is the high affinity IgE receptor, well known for its role in mediating activation of mast cells and basophils during acute allergic reaction. FceRI is also expressed in DCs, but its role in DCs has been elusive. We found that FceRI traffics differently in DCs from mast cells or basophils; FceRI was constitutively internalized in DCs while it stayed at cell surface in mast cells or basophils. This endocytic trafficking made FceRI in DCs mediate cellular entry of IgE and also made DCs promote clearance of serum IgE. This finding reveals a novel pathway of FceRI trafficking in DCs and provides mechanistic insight on the extremely short half-life of circulating IgE.
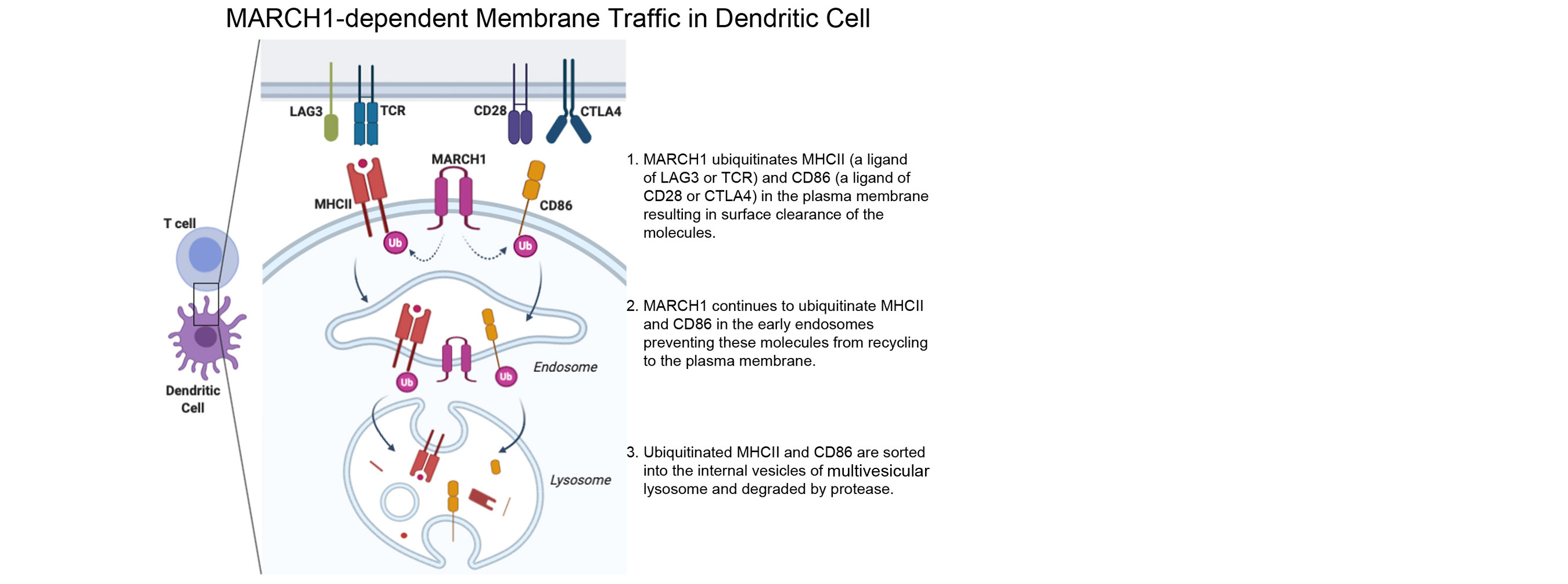
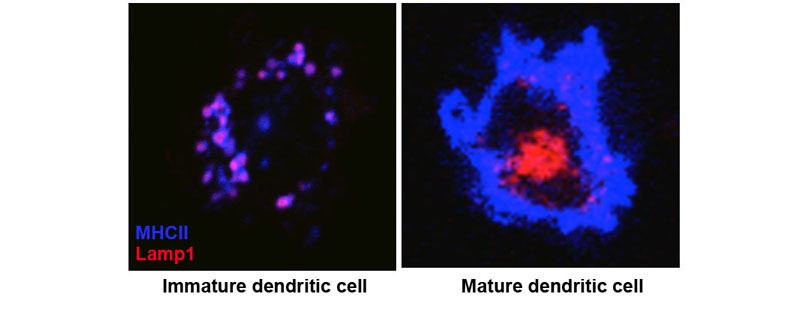
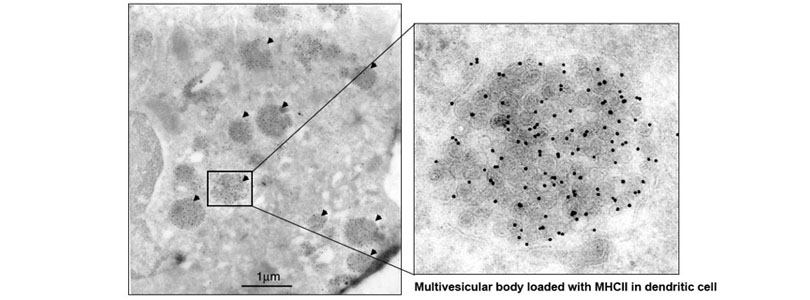
We also found that MHCII is constitutively endocytosed and degraded in the lysosomes. This endolysosomal trafficking was dependent on post-translational modification that involved attachment of a poly-ubiquitin to the evolutionarily conserved cytoplasmic lysine of the MHCII beta chain. The ubiquitin-dependent endocytosis of MHCII was crucial for DCs to maintain the physical and functional integrity of the plasma membrane microdomains named lipid raft or tetraspanin web. In the absence of the ubiquitin acceptor lysine of MHCII, these microdomains were overcrowded by MHCII, which in turn resulted in distortion of other membrane constituents including the adhesion molecule CD48 and the tetraspanin molecule CD9. Distortion of these molecules impaired DC ability to make stable conjugates with immature CD4+ thymocytes resulting in poor activation of these cells and failure to express Foxp3, the transcription factor that drives regulatory T (Treg) cell differentiation. This finding reveals that ubiquitin-dependent MHCII endocytosis is an important quality control mechanism by which DCs maintain the homeostasis of the plasma membrane domains that supports development of Treg cells.
Our current research focuses on a membrane-anchored ubiquitin ligase named MARCH1 (Membrane-Associated RING-CH1). MARCH1 is a transmembrane protein and the ubiquitin ligase that attaches a ubiquitin chain to MHCII. MARCH1 also mediates ubiquitination of CD86 and possibly other membrane proteins. However, the molecular mechanism underlying its enzymatic activity and its role in DC functions are not completely understood. Our major objectives are as following.
1. Determine the role of MARCH1 in DC function of driving T cell immunity.
2. Determine the role of MARCH1 in allergic asthma and autoimmune diseases.
3. Define MARCH1 interactome and structure.